Introduction
Paxlovid, the pill that has become the go-to treatment for COVID-19 treatment, was granted full approval in May by the Food And Drug Administration (FDA) for the treatment of mild-to-moderate COVID-19 in adults at high risk for severe disease, including hospitalization and death. The drug also remains available to everyone 12 and older (weighing at least 88 pounds) who has mild-to-moderate disease and is at high risk for severe disease under an FDA Emergency Use Authorization.
Paxlovid is an oral antiviral pill that can be taken at home to help keep high-risk patients from getting so sick that they need to be hospitalized. So, if you test positive for the coronavirus and you are eligible to take the pills, you can take them at home and lower your risk of going to the hospital.
The drug, developed by Pfizer, has a lot of positives: It had an 89% reduction in the risk of hospitalization and death in unvaccinated people in the Clinical Trial that supported the EUA, a number that was high enough to prompt the National Institutes of Health (NIH) to prioritize it over other COVID-19 treatments. Studies outside of the laboratory have since confirmed Paxlovid’s effectiveness among people who have been vaccinated. It’s cheaper than many other COVID-19 drugs (at this time, U.S. residents eligible for Paxlovid will continue to receive the medicine at no charge), and, perhaps most reassuring, it is expected to work against the Omnicron Variant.
“It’s really our first efficacious oral antiviral pill for this virus,” says Scott Roberts, MD, a Yale Medicine infectious diseases specialist. “It shows clear benefit, and it really can prevent hospitalization and death in people who are at high risk.”
FDA approval will allow Paxlovid to remain available for adults indefinitely. Meanwhile, Pfizer continues to gather pediatric data to submit for FDA approval in children at a future date.
As far as convenience, this medication is considered an improvement over treatments like remdesivir (approved by the FDA in October 2020), which is administered by intravenous (IV) injection. The FDA also granted an EUA in December to a pill from Merck called molnupiravir (Lagevrio), but some studies suggest that molnupiravir has only a 30% reduction in the risk for hospitalization and death from COVID-19.
Paxlovid working
| Mechanism of Action | Details |
| Active Ingredients | – Nirmatrelvir – Ritonavir |
| Protease Inhibition | – Paxlovid is an active 3Cl protease inhibitor. – It inhibits a necessary protease in the viral replication procedure. |
| Active Pharmaceutical Ingredient | – The active pharmaceutical ingredient in Paxlovid is PF-07321332. – PF-07321332 is a reversible covalent inhibitor of 3C-like protease (3CLpro). |
| Inhibition of 3CLpro | – PF-07321332 blocks the cysteine protease activity of 3CLpro. – It covalently binds to the catalytic cysteine residue of 3CLpro. |
| Prevention of Viral Replication | – Inhibiting 3CLpro prevents the virus from replicating. – This reduces the amount of virus in the body. |
| Combination with Ritonavir and Nirmatrelvir | – Paxlovid contains a combination of ritonavir and nirmatrelvir. – Ritonavir is a pharmacokinetic enhancer that helps increase the levels of nirmatrelvir in the body. |
| Clinical Trial | – The combination of ritonavir and nirmatrelvir in Paxlovid is under study in phase III of the clinical trial. |
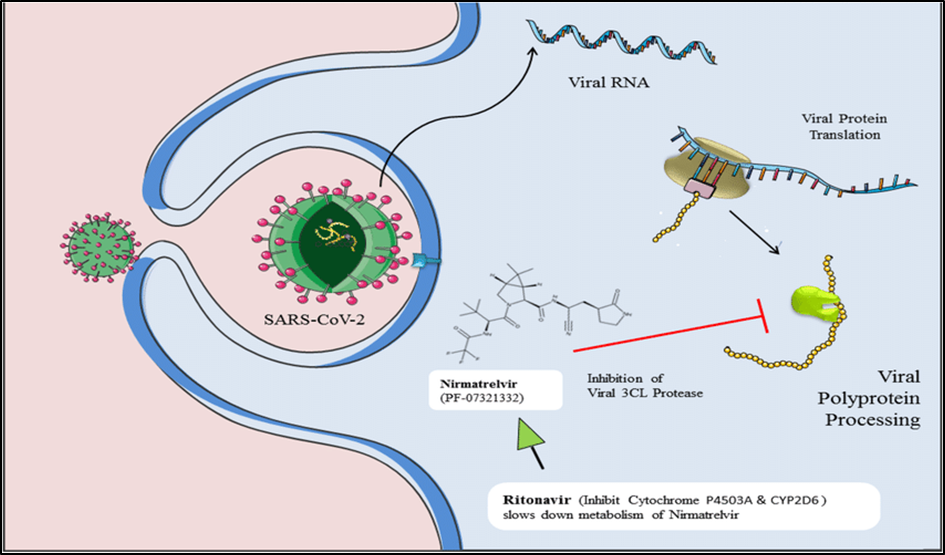
In summary, Paxlovid, with its active ingredients nirmatrelvir and ritonavir, works by inhibiting the 3C-like protease (3CLpro) necessary for viral replication. The active pharmaceutical ingredient PF-07321332 in Paxlovid acts as a reversible covalent inhibitor of 3CLpro, blocking its cysteine protease activity.
This inhibition prevents the virus from replicating and reduces the viral load in the body. Ritonavir, in combination with nirmatrelvir, enhances the pharmacokinetics of Paxlovid. The combination is currently being studied in phase III of the clinical trial.
Diagrammatic Representation of Mechanism of Action
Clinical Trials History
Paxlovid, an oral contraceptive developed by Pfizer, has undergone several clinical trials to determine its effectiveness and safety. Below is a summary of the clinical history of Paxlovid:
Phase I: A Phase I study in healthy adults was conducted to evaluate the safety and tolerability of Paxlovid. The study found that Paxlovid was well tolerated and did not cause serious side effects.
Phase II/III: A Phase II/III study of Paxlovid was conducted in non-hospitalized adults with COVID-19 at risk of developing severe illness. The study showed that people without the Paxlovid vaccine had 89% lower hospitalization and death rates. It also reduced hospitalization and death by 86% in the high-risk unvaccinated group, compared with 58% in the high-risk group.
Emergency Use Authorization: In 2021, the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization to allow the drug to treat mild to moderate cases of COVID-19 at risk of serious infection for COVID-19. Those considered at risk are those aged 50 and over; Adults or children aged 12 years and older with heart, kidney, and lung disease.
Phase III: The combination of ritonavir and nirmatrelvir in Paxlovid is in phase III clinical trials. This study was designed to evaluate the efficacy and safety of the combination in adults with non-hospitalized COVID-19 at high risk of severe disease.
As a result, Paxlovid has gone through several clinical trials to check its safety and effectiveness. The drug has been shown to be effective and efficient in reducing the risk of hospitalization and death in high-risk COVID-19 patients. The combination of ritonavir and nirmatrelvir in Paxlovid is currently in phase III clinical trials.
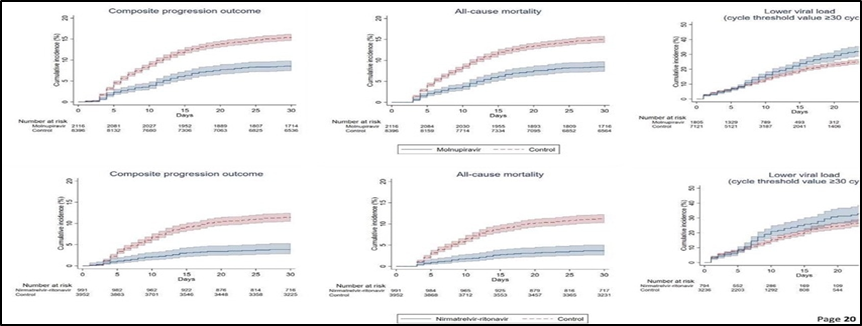
Efficacy Table of Paxlovid
Advantages
Paxlovid is an oral antiviral medication manufactured by Pfizer that can be taken at home to help high-risk patients avoid serious illness and the need for hospitalization. Some of the benefits of Paxlovid include:
Reduced risk of hospitalization and death: In clinical studies, Paxlovid reduced the risk of hospitalization and death by 89% in unvaccinated people. It also reduced hospitalization and death by 86% in the high-risk unvaccinated group, compared with 58% in the high-risk group.
As effective as early treatment: Paxlovid as early treatment is effective in preventing hospitalizations for COVID-19. It stops the virus that causes COVID-19 by balancing and reducing the number of bacteria in your body.
Results last up to six months: The effects of Paxlovid last up to six months after infection, according to a study published in JAMA Internal Medicine.
Effective in vaccinated and unvaccinated people: Non-laboratory studies have shown that Paxlovid is effective in vaccinated people. It affects unvaccinated people, people who have been vaccinated and then vaccinated, and people who have recovered from COVID-19 but have relapsed one or more times since.
Cheaper price than many other anti-covid-19 drugs: Currently, Paxlovid is cheaper than many other covid-19 drugs.
Effective and efficient: Paxlovid was well received and effective, reducing the risk of serious infections by 88% in clinical trials.
It contains two types of drugs (nimaprevir and ritonavir) that have been part of drugs used to treat HIV for decades, so this treatment has many years of research and treatment. Less likely to cause unwanted side effects: Chinese antiretroviral drugs appeared to be as effective as Paxlovid and cause fewer side effects in phase 3 trials. This new drug is less likely than Paxlovid to cause adverse side effects from its interaction with other drugs.
Disadvantages
Taste changes (“Paxlovid mouth”)-
In fact, it was the most reported side effect during clinical trials. It’s also a common side effect of ritonavir (Norvir) — one of the two antivirals in Paxlovid. But even though it’s typical, that doesn’t mean it’s pleasant.
Diarrhea-
It is a common side effect of many medications. While uncommon, loose stools are possible with Paxlovid. About 3% of clinical trial participants reported this side effect.
High blood pressure-
It is not unusual for a medication to raise blood pressure. And this can be a concern if you already have high blood pressure or other heart conditions. Small numbers of people who took Paxlovid have reported this side effect.
Headache-
Headaches are no fun and can make it difficult to complete your usual activities. Paxlovid may cause headaches for some people who take it. But it is relatively rare with the medication.
Liver damage-
As mentioned earlier, Paxlovid contains the medication ritonavir. Ritonavir is known to contribute to liver damage. This side effect is more likely to happen in people who already have liver problems.
Market Potential
The COVID-19 industry has seen significant growth in recent years as the outbreak of the disease has led to increased demand for diagnostics, treatments, and vaccines. Here are some details on the COVID-19 market size and potential:
The global COVID-19 testing market size is worth US$97.4 billion in 2021 and is expected to decline at a compound annual growth rate (CAGR) of 7.7% from 2022 to 2030. The global market size of COVID-19 tests is expected to be worth $8. It will reach $4 billion by 2027, with a 2.7% CAGR during the forecast period. The value of Global COVID-19 Diagnostics market size was US$46.76 billion in 2021.
Global COVID-19 Treatment Market size value at US$30.It will reach 7 billion in 2021 and is expected to grow with a CAGR of -8.3% from 2022 to 2031. The global diagnostic services market for
COVID-19 is expected to be worth $60.3 billion in 2020 and grow to $195.1 billion by 2025. Since the beginning of the global pandemic, the COVID-19 industry has seen a growing need for diagnosis, treatment and vaccines. The market size for COVID-19 diagnosis and treatment is expected to decline over the next few years, but the market for COVID-19 diagnostic services is expected to grow. Marketplace, Abbott, Roche, Pfizer, Merck etc. It’s multi-part with many players, including pharmaceutical giants.

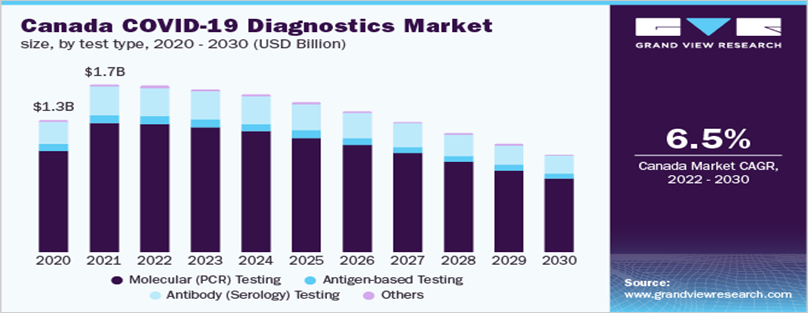
Diagnostic Market of Covid
COVID-19 Diagnostics Market Report Scope
| Report Attribute | Details |
| Market size value in 2022 | USD 95.2 billion |
| Revenue forecast in 2030 | USD 50.1 billion |
| Growth Rate | CAGR of (7.7%) from 2020 to 2030 |
| Base year for estimation | 2020 |
| Historical data | 2021 |
| Forecast period | 2022 – 2030 |
| Quantitative units | Revenue in USD million and CAGR from 2020 to 2030 |
| Report coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments covered | Product & service, sample type, test type, mode, end-use, region |
| Regional scope | North America; Europe; Asia Pacific; Latin America; MEA |
| Country scope | U.S.; Canada; Germany; U.K.; France; Spain; Italy; Russia; China; India; Japan; South Korea; Australia; Brazil; Mexico; South Africa; Saudi Arabia |
| Key companies profiled | Hologic Inc.; Thermo Fisher Scientific, Inc.; F. Hoffman-La Roche Ltd.; Perkin Elmer, Inc.; Veredus Laboratories; 1drop Inc.; ADT Biotech SdnBhd; Laboratory Corporation of America Holdings; bioMérieux SA; Danaher; Mylab Discovery Solutions Pvt. Ltd.; Neuberg Diagnostics; ALDATU BIOSCIENCES; Quidel Corporation; Quest Diagnostics; altona Diagnostics GmbH; Luminex Corporation; Abbott |
Market Growth
In the past few months, the focus of new entrants has shifted to in-house testing and integration of advanced software. This should make patients more comfortable during the use of laboratory tests. One of these new entrants is Change Health, which offers mobile services for home testing and commercial testing. As of September 2020, the Switch Health mobile quick test has used more than 30 mobile phones to diagnose people with coronavirus and has served more than 30 customers, including office workers and athletes.
The increase in patient numbers worldwide, the great demand for rapid diagnosis and the lack of specific drugs or vaccines are the main factors driving the Covid-19 diagnostic market.
According to World Meter, the total number of patients increased from 33,556,252 patients who tested positive on September 28, 2020 to 55,961,380 as of November 17, 2020. In addition, these tests will pave the way for the development of tourism and create a good environment for organizations working in the Covid-19 diagnostic sector. The tourism sector, which is the sector most affected by the epidemic process, caused an increase in the number of COVID-19 tests after August 2020, especially in countries and regions with economic activity. This should also enable PoC testing to enter the market because these tests can overcome the limitations associated with traditional testing.
Complex and pure reagents such as probes, enzymes and primers are required for tests in laboratories. A combination of factors such as urgent demand, export restrictions and inventories has led to a shortage of reagents. In addition, since these reagents are produced by a small business, the production capacity is not sufficient, causing shortages. The worldwide shortage-to-demand ratio of reagents is expected to negatively impact all diagnostics and hinder the growth of the Covid-19 diagnostic market.The Covid-19 pharmaceutical market is divided into two segments based on technology: PCR and Immunoassays.
With the increasing number of COVID-19 patients in 2020 and 2021, the government focused on preventing further spread of the disease, lack of treatment and vaccine, and the need for timely diagnosis, thereby expanding the market for COVID-19 diagnostics. Authorities are focusing on developing time-saving and accurate test kits to detect COVID-19. For example, in December 2021, F-Hoffman LaRoche Ltd. announced that it will produce CE marked SARS-CoV 2 and Flu A/B antigen rapid test kits in the market. Since the start of the global epidemic, the FDA has approved approximately 88 clinical trials and 256 molecular tests, making up more than 350 of the total approved tests.
Conclusion
In summary, Paxlovid, an oral antibiotic produced by Pfizer, reduces the amount of virus that causes COVID-19 in the body and prevents adverse symptoms. Clinical trials have shown that the drug is effective and effective in reducing the risk of serious illness by 88%. The active ingredient in Paxlovid is nirmatrelvir, an oral protease inhibitor developed by Pfizer to inhibit major protease (Mpro). The drug has gone through many clinical trials to check its safety and efficacy and has been approved by the FDA for emergency use. The combination of ritonavir and nirmatrelvir in Paxlovid is currently in phase III clinical trials.
The COVID-19 industry has seen significant growth in recent years as the outbreak of the disease has led to increased demand for diagnostics, treatments and vaccines. The market size for COVID-19 diagnosis and treatment is expected to shrink in the coming years, but the market for COVID-19 tests is expected to grow. Paxlovid’s performance record looks promising despite competition from other anti-Covid-19 drugs, and Pfizer has pushed manufacturing capacity to meet demand for the drug.
Paxlovid’s marketing reports are very promising, with the drug showing good results in clinical trials and reducing the risk of serious infections by 88%. The drug has gone through several clinical trials to confirm its safety and efficacy and has been approved by the FDA for emergency use. The active ingredient in Paxlovid is nirmatrelvir, an oral protease inhibitor specifically designed by Pfizer to inhibit the major protease (Mpro). The competition between paxlovid and molnupiravir highlights the importance of effective and effective treatment against COVID-19. Despite competition from other COVID-19 drugs, Pfizer has pushed manufacturing capacity to meet demand for the drug.
The increased availability of drugs will help meet the growing need for effective treatment for COVID-19, especially if the disease continues to spread throughout the global community. As a result, Paxlovid’s market potential is vast, the drug has been shown to be effective in reducing the risk of serious disease, and Pfizer is committed to meeting demand for the drug.
References
- American Society for Clinical Pharmacology and Therapeutics. (2023). DDI potential with Paxlovid was evaluated using US Prescribing and DailyMed Information or relevant literature for each drug. Retrieved from https://ascpt.onlinelibrary.wiley.com/doi/10.1002/cpt.2835
- Chakraborty, S., & Banerjee, S. (2022). Paxlovid: A promising drug for the challenging treatment of SARS-COV-2 in the pandemic era. PMC. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10043822/
- Pfizer. (n.d.). Understanding the science behind PAXLOVID. NextGen Global Brand site. Retrieved from https://www.paxlovid.my/mechanism-of-action
- News Medical. (2023). Study confirms the benefit of Paxlovid as an early-stage treatment to prevent COVID-19 hospitalization. Retrieved from https://www.news-medical.net/news/20230317/Study-confirms-the-benefit-of-Paxlovid-as-an-early-stage-treatment-to-prevent-COVID-19-hospitalization.aspx
- PubMed. (2022). Paxlovid: Mechanism of Action, Synthesis, and In Silico Study. Retrieved from https://pubmed.ncbi.nlm.nih.gov/35845944/
- PubMed Central. (2022). Paxlovid: Mechanism of Action, Synthesis, and In Silico Study. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9283023/
- https://www.grandviewresearch.com/industry-analysis/covid-19-diagnostics-market






Leave a Reply