Several immune cells, including T cells and natural killer (NK) cells, contain the protein receptor known as TIGIT ( T-cell immunoglobulin and ITIM domain). It is a member of the family of co-inhibitory receptors, which also includes the T-cell response regulators PD-1, CTLA-4, and LAG-3.
It controls immunological responses and keeps the immune system from becoming overly activated. TIGIT has become a promising target for immunotherapy in the setting of cancer.
Functioning of TIGIT
TIGIT functions as an immunological checkpoint receptor, which means it interacts with its ligand, CD155, on various immune cells and tumour cells, to control immune responses. TIGIT and CD155’s interaction produces inhibitory signals that reduce immune cell activation and performance, stifling anti-tumor immune responses.
TIGIT starts a signalling cascade inside the immune cell when it binds to CD155. This cascade also involves the recruitment of tyrosine phosphatases, which dephosphorylate important signalling molecules involved in the activation of immune cells, as well as the activation of inhibitory pathways. The end result of this mechanism is the suppression of cytokine synthesis, proliferation, and cytotoxicity—all immune cell effector activities.
TIGIT and CD155 on immunological and tumour cells interact to provide an immunosuppressive milieu in the context of cancer. Tumour cells have the ability to increase the expression of CD155, which efficiently activates TIGIT on immune cells and reduces their capacity to produce a successful anti-tumor response. Tumours can continue to develop while evading immune surveillance thanks to this immune evasion mechanism.
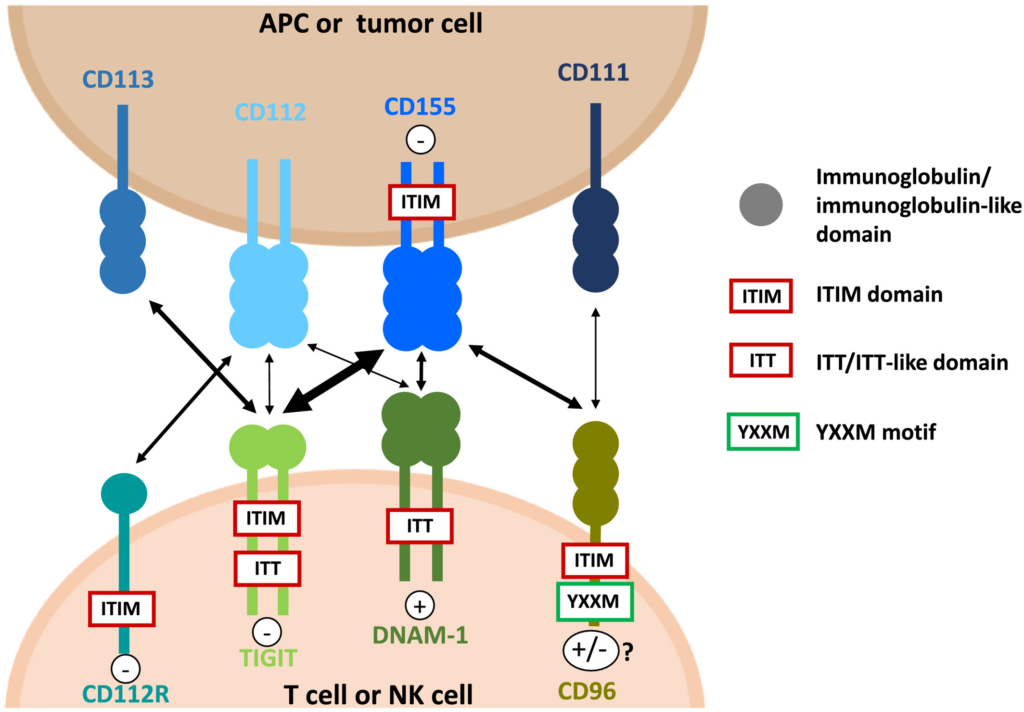
TIGIT can be addressed utilising a variety of treatment modalities to counteract this immunosuppressive effect and improve anti-tumor immune responses. The link between TIGIT and CD155 can be blocked by monoclonal antibodies that selectively bind to TIGIT. This stops the inhibitory signalling and restores the activation and functionality of immune cells. Immune cells are better able to identify and target tumour cells as a result of this blockage.
TIGIT-targeted treatments can also improve anti-tumor immune responses when combined with other immunotherapies, like checkpoint inhibitors. To unblock the immune system and encourage an immunological response against cancer cells, checkpoint inhibitors also target additional inhibitory receptors, such as PD-1 or CTLA-4. TIGIT blocking with checkpoint inhibitors may work in synergy to enhance the effectiveness of treatment.
In general, TIGIT-targeted therapies seek to interfere with the connection between TIGIT and CD155, lessen immune repression, and encourage a strong anti-tumor immune response. The effectiveness, safety, and ideal combinations of TIGIT-based medicines in treating various cancer types are now being studied in ongoing studies and clinical trials.
Viability
TIGIT has demonstrated promise as a therapeutic target for several cancer types. The effectiveness of TIGIT-targeted medicines in treating many cancer types has been examined in preclinical and clinical research, including:
Solid Tumours: TIGIT-based treatments have been looked at for solid tumours such lung, breast, ovarian, colorectal, melanoma, and more. TIGIT inhibitors have shown promising results in clinical trials, especially when combined with other immunotherapies or conventional medicines.
Haematological Malignancies: The use of TIGIT inhibition in haematological malignancies, such as lymphomas and leukaemias, has also been studied. Studies have looked into the therapeutic potential of TIGIT antibodies and CAR-T cell therapies that target TIGIT. These cancers have also displayed encouraging activity, according to preliminary findings.
Clinical trials examining TIGIT-targeted treatments have occasionally had disappointing outcomes or showed only modest efficacy. It’s crucial to remember that clinical trial results can differ, and poor outcomes in some trials do not always mean that TIGIT-based medicines are useless in general. For example, TIGIT has not yielded positive results in lunge cancer studies but worked in other cases.
It’s important to note that because the field is still developing, unfavourable findings from specific trials do not exclude the possibility of TIGIT-targeted medicines entirely. To improve treatment plans, pinpoint ideal pairings, and identify the patient populations that would benefit most from TIGIT-based medicines, further research and clinical trials must be conducted.
Pharmaceutical Reach
Pharmaceutical companies have been inspired to research TIGIT inhibitors by the potential preclinical success of targeting TIGIT in cancer. The US FDA has designated Tiragolumab, a new cancer immunotherapy that targets TIGIT, as a breakthrough medicine in combination with Tecentriq for the treatment of non-small cell lung cancer, according to a January 2021 announcement from Genentech.
Another one of the most cutting-edge TIGIT inhibitors now undergoing clinical research is Tiragolumab. Ociperlimab, Vibostolimab, ASP-8374, AGEN1777, HLX301, and COM902 are the other potential TIGIT inhibitors under clinical research. These inhibitors are anticipated to enter the market throughout the forecast period after exhibiting positive results in clinical trials.
For the rights to the phase 1 anti-TIGIT monoclonal antibody EOS-448, GlaxoSmithKline is paying iTeos Therapeutics $625 million up front. The agreement positions GSK to compete with Bristol Myers Squibb, Gilead Sciences, Merck, and Roche for the hot immuno-oncology market niche.
Over the past 18 months, interest in TIGIT has surged, with Bristol-Myers and Gilead negotiating significant deals to enter the race and Merck and Roche rapidly extending their clinical development programmes. In order to secure EOS-448, GSK has now cobbled together an even larger upfront payment, spending more than Bristol-Myers and Gilead put together.
By paying $625 million up front, GSK is granted the opportunity to co-commercialize EOS-448 in the United States and get an equal share of the revenue. GSK has an exclusive licence elsewhere and will pay iTeos. Patients with advanced solid tumours are currently participating in a phase 1 dose-finding experiment with EOS-448.
The development of TIGIT-targeted treatments is being done by a number of pharmaceutical and biotechnology firms. The businesses that have been actively engaged in TIGIT-related research and clinical trials are listed below:
Genentech/Roche: As a part of Roche, Genentech has been at the forefront of TIGIT research and has created the TIGIT inhibitor tiragolumab (also known as RG6058). Clinical trials have assessed tigolumab, including ones in which it was combined with the PD-L1 inhibitor atezolizumab.
Merck & Co.: Merck has taken part in studies and clinical trials pertaining to TIGIT. They have created the TIGIT inhibitor MK-7684 and run clinical trials to see how well it works when combined with the PD-1 inhibitor pembrolizumab.
BMS-986207 is a TIGIT inhibitor that Bristol Myers Squibb (BMS) has created. BMS has been involved in TIGIT research. Its effectiveness and safety in combination with nivolumab (a PD-1 inhibitor) have been tested in clinical trials.
Agenus: Agenus is an immuno-oncology-focused biotechnology business. An anti-TIGIT antibody they created called AGEN1777 has been tested in clinical studies both alone and in combination with existing immune checkpoint inhibitors.
Another biotechnology business actively engaged in TIGIT research is Arcus Biosciences. They created AB154, an anti-TIGIT antibody, which has been tested both alone and in conjunction with other immunotherapies in clinical trials.
Surface Oncology is a biopharmaceutical business that has been working on the development of the anti-TIGIT antibody SRF617 and clinical research is ongoing.
In the field of cancer immunotherapy, TIGIT-targeted treatments have demonstrated promise and potential for advancement. The following elements point to the development of TIGIT-targeted therapies:
Early findings from preclinical and clinical studies have shown that TIGIT-targeted treatments can improve anti-tumor immune responses. Positive outcomes from clinical trials imply that TIGIT inhibition has the potential to enhance therapeutic outcomes for specific cancer types, particularly when used in conjunction with other immunotherapies.
Unmet Medical Need: Despite important developments in the treatment of cancer, there is still a demand for efficient medicines for a variety of tumour types. As a novel immunotherapeutic target, TIGIT gives a different way to boost anti-tumor immune responses and overcome immune suppression.
Combination Therapy Strategies: TIGIT-targeted medicines have demonstrated promise. Combining TIGIT-targeted treatments with other immunotherapies, such as PD-1/PD-L1 inhibitors or CTLA-4 inhibitors, has been investigated. Immune checkpoint inhibitor combinations have the potential to enhance therapeutic outcomes and have synergistic effects. More research into the best TIGIT inhibitor combo techniques may result in greater efficacy and more widespread applicability.
Expansion of Ambit to Different Cancer Types: Although early research concentrated on solid tumours, current studies are investigating the possibility of TIGIT-targeted therapy in haematological malignancies, such as lymphomas and leukaemias. If effective, the application of TIGIT inhibition to further cancer types may have a considerable impact on its development and uptake.
Research and Development: Pharmaceutical businesses and academic centres are actively working to understand TIGIT and create new treatments. The significance of TIGIT in cancer immunity will be better understood as a result of ongoing research initiatives, including clinical trials, which will also help pinpoint the most effective treatment options. The development potential of TIGIT-targeted medicines is aided by this ongoing research and development.
Although TIGIT-targeted medicines have the potential to increase, it’s crucial to remember that their effectiveness will depend on a variety of variables, including the results of clinical trials, regulatory approvals, market competition, and the overall development of cancer immunotherapy. The future growth potential of TIGIT-targeted medicines will be clarified with careful field observation and additional research.
References:
https://pubmed.ncbi.nlm.nih.gov/32900861/
https://www.frontiersin.org/articles/10.3389/fonc.2022.1091782/full
https://jitc.bmj.com/content/8/2/e000957
https://encyclopedia.pub/entry/8520
https://en.wikipedia.org/wiki/TIGIT
https://www.biospace.com/article/tigit-inhibitor-drug-clinical-trials-report-2022/






Leave a Reply