Roctavian, a gene therapy product developed by BioMarin, has been approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of hemophilia A(It is a form of bleeding). A non-coagulating protein called factor VIII. Roctavian contains the active ingredient “valoctocogene roxaparvovec”, which causes the seeds to be delivered to the body. Roctavian’s approval was hailed as a major breakthrough in the treatment of hemophilia A, as it was the first and only gene therapy to treat hemophilia A.
BioMarin is focused on commercializing Roctavian, which includes developing enough Roctavian to meet the needs of the business over the life of the product, educating doctors and patients about Roctavian, and ensuring that all adults benefit from Roctavian therapy. The company is also investigating Roctavian, which offers guaranteed benefits to all US payers, and is examining similar regulations in other countries.
Roctavian’s market potential is huge because hemophilia A is a rare disease and Roctavian has been designated as an orphan drug.Roctavian’s approval is a huge success for BioMarin, which is currently facing the challenge of commercializing the product. The company is focusing on Roctavian’s results-driven responsibility as a unique way of doing business, and it is not yet clear how this strategy will succeed.However, the benefits of Roctavian in patients with severe hemophilia A are significant, and successful marketing of Roctavian could have a significant impact on the treatment of rare diseases.
BioMarin focuses on commercializing Roctavian, including developing enough Roctavian to meet industry needs over the life of the product, educating doctors and patients about Roctavian, and ensuring that all adults eligible for Roctavian receive the treatment. 21. Orphan Pharmaceuticals The approval of Roctavian is hailed as a huge success for BioMarin, which is currently focused on commercializing the product.
Mechanism of Action – Roctavian

Roctavian is a gene therapy for the treatment of severe hemophilia A, a bleeding disorder caused by a deficiency of a blood clotting protein called Factor VIII. Roctavian contains the active drug valoctocogene roxaparvovec, the adeno-associated virus serotype 5 (AAV5)-based gene therapy vector. In doing so, Roctavian restores FVIII production, reducing the risk of bleeding while reducing or eliminating the need for doses of active Factor VIII, hemophilia A Roctavian is an advanced therapy. A drug called a gene therapy product that works by delivering genes to the body. The active ingredient in “Roctavian, valoctocogene roxaparvovec”, is adeno-associated virus serotype 5 (AAV5) Section
In conclusion, Roctavian is a therapeutic product indicated for the treatment of hemophilia. Roctavian’s method of working involves delivering short but active F8 genes to liver cells, the main producer of blood clots. Body. In doing so, Roctavian restores active FVIII production, reducing the risk of bleeding while reducing or eliminating the need for frequent VIII infusions.
Advantages of Roctavian
Roctavian is a gene therapy product developed by BioMarin that is more effective in the treatment of hemophilia A. Here are some of the benefits of Roctavian: hemophilia A This is in contrast to conventional treatments that require frequent. Factor VIII infusions to prevent bleeding events.
Reduced Need for Factor VIII Infusions: Roctavian can reduce or potentially eliminate the need for frequent Factor VIII infusions, which is the current standard of care for patients with hemophilia A, as they will not need multiple infusions.
Ability to prevent bleeding: Roctavian has been shown to prevent bleeding in patients with severe hemophilia A and requires up to six years of preventive treatment.This is a significant advantage because bleeding is the cause of pain and causes severe pain.
Transformative Potential: Roctavian has the potential to change the way healthcare professionals and patients in the community care for bleeding disorders The commercial success of Roctavian could have a major impact on the treatment of hemophilia A.
Orphan Drug Designation: Roctavian has Orphan Drug Designation in the US and Europe to accelerate clinical development and clinical management.This selection demonstrates Roctavian’s ability to address unmet medical needs in the treatment of hemophilia A.
In conclusion, Roctavian has several advantages over existing hemophilia A treatments. Frequent Factor VIII infusions prevent bleeding and transform the physician and patient population. cf. bleeding care. Additionally, Roctavian has been designated as an orphan drug.
Disadvantages of Roctavian
Roctavian, a gene therapy product produced by BioMarin, has many negative side effects. Below are some of the limitations and uncertainties associated with Roctavian.
Uncertain and limited benefits: According to the European Medicines Agency (EMA), there are uncertainties and limitations to the benefits of Roctavian. This may be due to the limited data available on the long-term safety and efficacy of Roctavian.
High cost: Roctavian is a high cost treatment with $2.9 million per patient. Such a high price would limit the use of Roctavian in patients with hemophilia A.
Limited Data on the Efficacy of Residual Disease Tests: According to research by the Pharmacy Times, there are no data on Roctavian-associated residual disease tests. This will limit our understanding of Roctavian’s long-term safety and efficacy.
Limited data on patients with inhibitors: Roctavian has only been studied in patients without the corresponding VIII inhibitors (antibodies).
Uncertainty about long-term safety and efficacy: There is uncertainty about the long-term safety and efficacy of Roctavian as the treatment is new and its long-term effects are limited
As a result, Roctavian has some potential. Disadvantages include the uncertainty and limitation of positive results, high cost, limited data to assess the effect of residual disease, limited data in patients with inhibitors, and uncertainty about long-term safety and efficacy. While Roctavian has the potential to revolutionize the treatment of severe hemophilia A, these limitations and uncertainties should be considered when evaluating treatment.
Clinical Phase History and Nods
| Clinical Trial Phase | Description |
| Phase 1/2 trial | The safety and efficacy of increasing doses of Roctavian have been evaluated in patients with hemophilia A. Results showed that a single dose of Roctavian prevented bleeding and required up to six years of anticoagulation in patients with hemophilia A. |
| Phase 3 trial | The largest and longest Phase 3 clinical trial in gene therapy for hemophilia, called GENER8-1, supported FDA and EMA approval of Roctavian for hemophilia A. Results showed that patients treated with Roctavian had a 52% reduction in annual bleeding. |
| FDA approval | Roctavian was approved by the FDA in June 2023 to treat adults with hemophilia A. Approval was supported by positive results from the GEENer8-1 study, which showed a reduction in bleeding events in patients treated with Roctavian. |
| EMA approval | Roctavian was approved by the EMA for the treatment of hemophilia A in 2022. Approval is based on the results of the GEENer8-1 study, which showed a reduction in bleeding events in patients treated with Roctavian. |
| Orphan drug designation | Roctavian will receive EMA approval in 2022 for the treatment of hemophilia A. Approval is based on the results of the GEENer8-1 study, which showed a reduction in bleeding events in patients treated with Roctavian. |
Taken together, results from clinical trials of Roctavian have been shown to be effective in reducing bleeding events in patients with hemophilia A.
Market Potential of Roctavian
Roctavian has the potential to reverse the treatment of severe hemophilia A, a rare bleeding disorder caused by a deficiency of a blood clotting protein called Factor VIII. Roctavian’s market potential is huge because hemophilia A is a rare disease and Roctavian has been designated as an orphan drug. Roctavian approval was hailed as a huge success for BioMarin, the company now focused on product commercialization
Roctavian’s manufacturer, BioMarin, is now focusing on Roctavian’s business, including producing enough Roctavian to meet demand. All lifecycle companies are required to educate physicians and patients about Roctavian and ensure access to treatment for all eligible adults dealing with Roctavian.
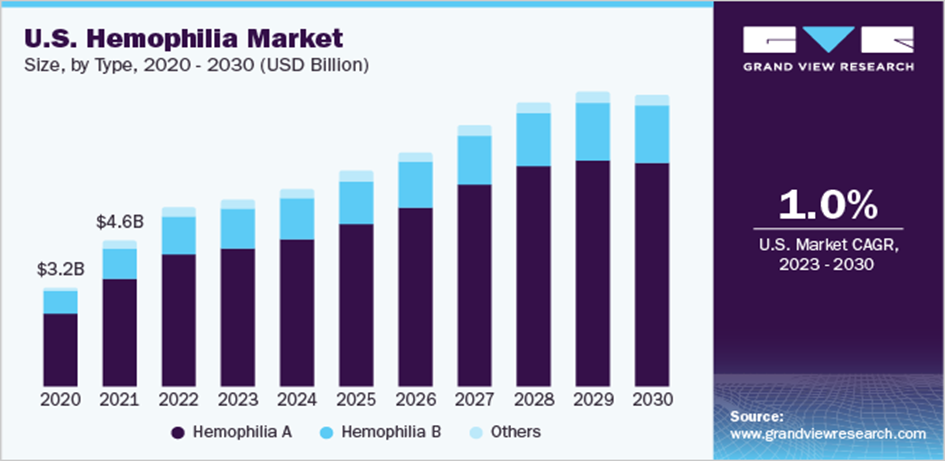
The global hemophilia treatment market size will be $11.1 billion with an expected annual growth rate (CAGR) of 5 in 2020. 9% growth between 2021 and 2028. The report also mentions that the increase in the incidence of hemophilia A and B and the increasing demand for advanced therapy are driving the growth of the market.
Consequently, the market potential for Roctavian is very large due to hemophilia It was selected to be Roctavian, a rare disease. drug orphan. The global hemophilia treatment market is expected to grow as the increase in hemophilia types A and B.
Market Players of Roctavian

There is only one company responsible for the production and sales of Roctavian, and that is BioMarin. Roctavian’s manufacturer, BioMarin, is now focusing on commercializing Roctavian, including producing enough Roctavian to meet commercial needs over the life of the product, informing doctors and patients about Roctavian, and ensuring everyone is satisfied with Roctavian Elderly. Treatment. When Roctavian launches, it will mostly be treated in medical facilities conforming to the 340B design and drastically reduced there, according to Forbes. BioMarin paid Roctavian a one-time settlement fee of $2.9 million. BioMarin said it expects the Roctavian to cost around €1 net 5 million ($1.6 million)
In a nutshell, there is only one player in the market for the manufacture and sale of Roctavian, and that is BioMarin. BioMarin acquired the Roctavian business and paid a one-time settlement fee of $2.9 million. After Roctavian is announced, it will only be treated at 340B compatible clinics and will be greatly discounted in price.
Market Value of Roctavian (2023 -2030)
| Year | Description |
| 2023 | Roctavian is priced at $2.9 million per patient. |
| 2023 | BioMarin cut its annual sales forecast range for Roctavian to $50 million to $100 million, from $100 million to $200 million |
| 2023 | BioMarin has said it expects Roctavian’s net price to be about €$1.5 million ($1.6 million |
| 2028 | The global hemophilia treatment market size is expected to grow at a compound annual growth rate (CAGR) of 5.9% from 2021 to 2028 |
| 2030 | The market potential for Roctavian is significant, as hemophilia A is a rare disease, and Roctavian was designated an orphan medicines |
As a result, Roctavian is priced at $2.9 million per patient, and BioMarin has reduced its annual sales for Roctavian to the $50 million to $100 million range. BioMarin said it expects Roctavian to net about 1.5 million euros ($1.6 million).
The global hemophilia treatment market is expected to grow at a compound annual growth rate (CAGR) of 5.9% from 2021 to 2028. The market potential for Roctavian is huge because hemophilia A is a rare disease and Roctavian is defined as an orphan.
Comparison of Total Haemophilia Market
| Report Attribute | Details |
| Market size value in 2023 | USD 13.53 billion |
| Revenue forecast in 2030 | USD 21.07 billion |
| Growth rate | CAGR of 6.6% from 2023 to 2030 |
| Base year for estimation | 2022 |
| Historical data | 2018 – 2021 |
| Forecast period | 2023 – 2030 |
| Report Updated | July 2023 |
| Quantitative units | Revenue in USD Billion and CAGR from 2023 to 2030 |
| Report coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments covered | Type; treatment type; therapy; distribution channel; region |
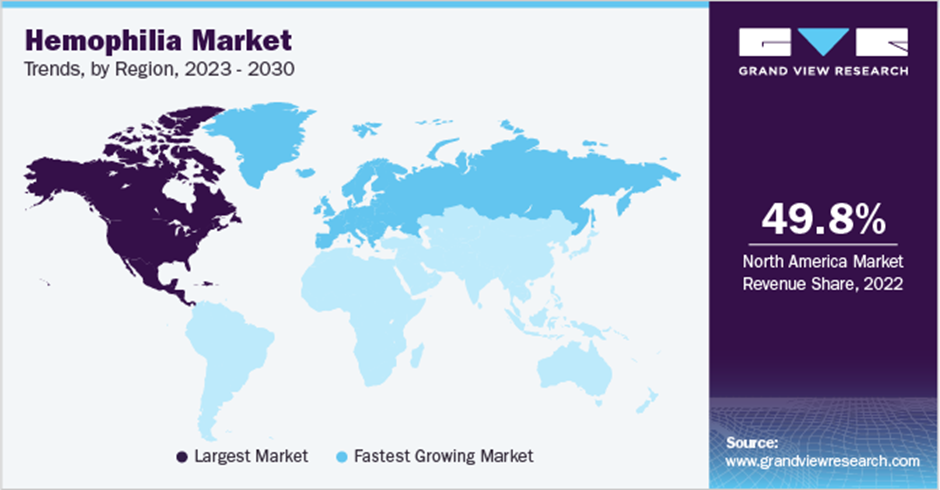
| Regional scope | North America; Europe; Asia Pacific; Latin America; MEA |
| Country scope | U.S.; Canada; Germany; UK; France; Italy; Spain; Denmark; Sweden; Norway; China; Japan; India; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; South Africa; Saudi Arabia; UAE; Kuwait |
| Key companies profiled | Takeda Pharmaceutical Company Limited; CSL Behring; Pfizer, Inc.; Bayer AG; BioMarin, Spark Therapeutics, Inc.; Sanofi; F. Hoffmann La-Roche Ltd..; Novo Nordisk A/S.; and Octapharma AG. |
| Customization scope | Free report customization (equivalent up to 8 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope. |
Hemophilia Market
Key Companies in Hemophilia Market
- Takeda Pharmaceutical Company Limited
- CSL Behring
- Pfizer, Inc.
- Bayer AG
- BioMarin
- Spark Therapeutics, Inc.
- Sanofi
- F. Hoffmann La-Roche Ltd.
- Novo Nordisk A/S.
- Octapharma AG.
Conclusion
In conclusion, Roctavian, a gene therapy product developed by BioMarin, has the potential to revolutionize the treatment of hemophilia A. FDA and EMA approval for Roctavian’s treatment for severe hemophilia A is a clinical milestone. less disease Roctavian is a one-time treatment that can reduce or eliminate the need for frequent Factor VIII infusions, prevent bleeding, and revolutionize the way the medical and patient care communities work. However, there are uncertainties and limitations regarding the long-term safety and efficacy of Roctavian, and more data are needed to understand its benefits and limitations. The high cost of Roctavian may also limit the access to treatment for people with hemophilia A.
Roctavian’s manufacturer, BioMarin, focused on marketing Roctavian and paid a one-time treatment fee of $2.5 million with discounts at clinics eligible for 340B. The global market for hemophilia treatment is expected to grow as the increase in hemophilia A and B and the increasing need for therapy drive market growth. BioMarin lowered Roctavian’s annual sales forecast from $50 million to $100 million, but the revised Roctavian revenue forecast includes contributions from Europe, the US and other markets, including selected patients. Garanti can help gene therapy overcome payment issues as BioMarin returns to Roctavian’s business.
The successful marketing of Roctavian may have a significant impact on the treatment of hemophilia A, but it is important to monitor long-term and effective treatment. Overall, Roctavian represents a significant advance in the treatment of severe hemophilia A, and physicians and patients should carefully consider its benefits and limitations.
References
- Assessment report – Roctavian – European Medicines Agency
- When Launched, Gene Therapy Roctavian Will Be Administered Mainly In 340B-Eligible Treatment Centers, Where It Will Be Substantially Discounted In Price – Forbes
- Roctavian (valoctocogene roxaparvovec-rvox) – CenterWatch
- Roctavian Receives Approval by the FDA for Severe Hemophilia A – HCPLive
- BioMarin’s Roctavian Cuts Bleeds, Draws Interest From Payers – Bloomberg
- Roctavian (formerly Valrox/BMN 270) – Hemophilia News Today
- BioMarin Announces Record-Breaking First Quarter 2023 Results
- BioMarin’s Roctavian Receives Approval from European Commission for Severe Hemophilia A
- Roctavian (valoctocogene roxaparvovec) – Hemophilia Federation of America
- Roctavian – Gene Therapy for Hemophilia A






Leave a Reply