LITFULO (ritlecitinib) capsules, manufactured by Pfizer, is a kinase inhibitor drug recently approved by the FDA for the treatment of alopecia areata (AA) in adults and adolescents. This agreement is most important in combating hair loss and providing people with new treatment options.
Ritlecitinib capsules are formulated with Ritlecitinib tosylate, a kinase inhibitor, as a white to off-white to pale red product. Capsules available in 50 mg immediate-release form for oral administration. Each capsule contains 50 mg of Ritlecitinib (equivalent to 80 mg) Contains various inactive ingredients such as 13 mg Ritlecitinib tosylate), crospovidone, glyceryl dibehenate, lactose monohydrate, microcrystalline cellulose and hypromellose (HPMC) capsule shell. The capsule shell is yellow/blue and opaque and has a bright blue FCF – FD&C Blue hypromellose, titanium dioxide and yellow iron oxide. It is worth noting that the Ritlecitinib comes with some warnings and precautions. Serious morbidity, mortality, cancer, adverse cardiovascular events (MACE) and thrombosis are risk factors associated with the use of Litfulo.In addition, the US Food and Drug Administration (FDA) approved Medication Guide also provides important information for patients. FDA approval of Ritlecitinib for the treatment of severe alopecia areata is an important first step in solving the problem. The advent of oral therapy, especially for pediatric alopecia, offers new possibilities for alopecia management and possibly improving the quality of life of affected individuals.
As a result, Lutiflo(ritlecitinib) capsules, manufactured by Pfizer, have been approved by the FDA as an oral treatment for severe alopecia areata in adults and adolescents. This agreement is most important in combating hair loss and providing people with new treatment options. The formulation and related warnings and precautions are listed in the Medication Information and Medication Guide. Additional information can be obtained from Pfizer’s clinical information and FDA approval letter.
Mechanism of Action
Ritlecitinib is a kinase inhibitor. Ritlecitinib irreversibly inhibits Janus kinase 3 (JAK3) and tyrosine kinases expressed in the hepatocellular carcinoma (TEC) kinase family by blocking the adenosine triphosphate (ATP) binding site. In a cellular context, ritlesitinib inhibits cytokine-induced STAT phosphorylation mediated by JAK3-dependent receptors. In addition, ritlecitinib inhibits immune receptor signaling that is dependent on members of the TEC kinase family. It is unclear whether inhibition of JAK or TEC family enzymes is associated with clinical efficacy.
Pharmacodynamics
Lymphocyte Subsets,Dose-related early reductions in absolute lymphocytes, T lymphocytes (CD3) and T lymphocyte subsets (CD4 and CD8) have been associated with Ritlecitinib therapy in patients with alopecia areata. In addition, NK cells (CD16/56) decreased at early doses and remained stable at low levels until week 48. For the 50 mg dose of QD, the mean lymphocyte baseline decreased but remained effective through week 48. No change in B lymphocytes (CD19) were observed in any treatment group.
Cardiac Electrophysiology
There is no clinical effect on the QTc interval in patients with alopecia areata 12 times the maximum dose of 50 mg once daily.
Pharmacokinetics
Ritlecitinib AUC0-tau and Cmax increased at approximately the same dose of 200 mg. Steady state is reached around the Sun.
Absorption
The actual oral bioavailability of ritlecitinib is approximately 64%. Ritlecitinib reaches its maximum plasma concentration within 1 hour after oral administration.
Effects of Food
Food had no significant effect on ritlecitinib exposure.Co-administration of 100 mg ritesitinib capsules with a low-fat meal decreased ritesitinib Cmax by approximately 32% and increased AUCinf by 11%. In clinical studies, ritlesitinib was administered without food
Distribution
Approximately 14% of circulating ritlesitinib is bound to plasma proteins.
Withdrawal
Ritlecitinib has a mean terminal half-life of 1.3 to 2.3 hours.
Ritlecitinib metabolism is mediated by several pathways, none of which contribute more than 25% of total metabolism. These mechanisms include:
Ritlecitinibis a drug whose mechanism of action involves the activity of neurotransmitters in the brain. It acts as a selective serotonin reuptake inhibitor (SSRI), increasing the level of serotonin found in the synaptic cleft. By doing this, Ritlecitinibimproves the transmission of serotonin, which is thought to reduce symptoms of depression and anxiety. Its exact mechanism and effects on other neurotransmitters are still under investigation.
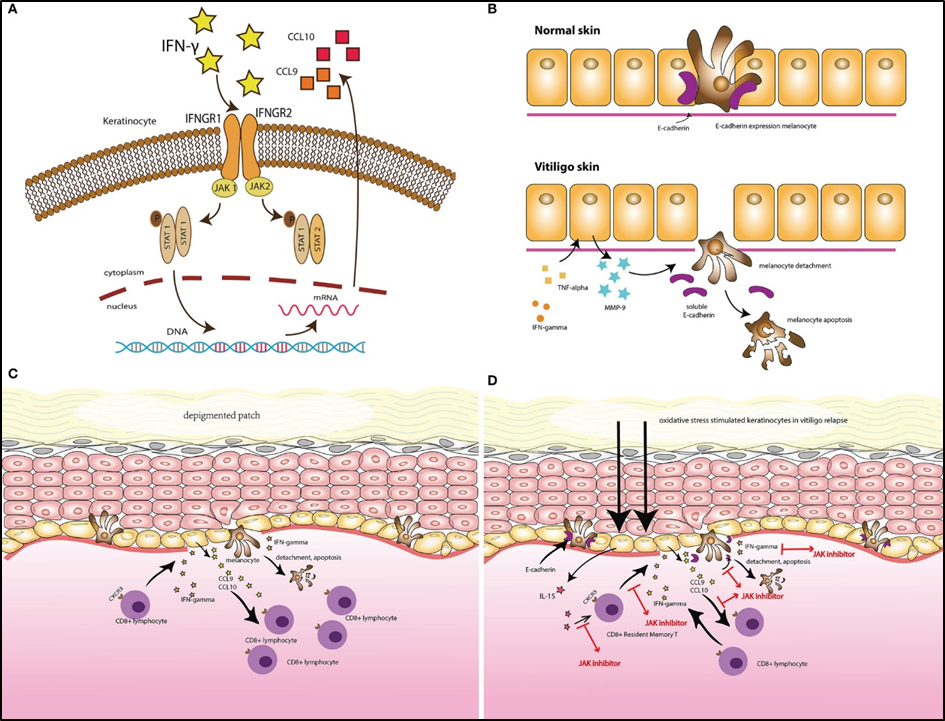
Mechanism of action of LITFULO™ (ritlecitinib)
Advantages of LITFULO (ritlecitinib)
Ritlecitinib is a promising drug in the treatment of alopecia areata and nonsegmental vitiligo. Here are some advantages of using Ritlecitinib:
Selective Inhibition: Ritlecitinib is a selective JAK inhibitor, meaning it specifically targets the JAK family of enzymes involved in cytokine and growth signaling pathways. Ritlesitinib selectively inhibits JAK1 and JAK3, avoiding the treatment of non-selective JAK inhibitors such as elevated cholesterol and liver enzymes, thrombocytopenia, and anemia. This makes Ritlecitinib a safer and more effective treatment for patients.
Treatment: Ritlecitinib is used orally, making it an immunosuppressive treatment in alopecia areata and vitiligo. This makes the difference between cosmetics and phototherapy, which is limited to the surface of the skin and may not be effective in preventing infection or causing complications.Treatment with ritlecitinib has the ability to regenerate and prevent infection in patients with vitiligo and to promote hair regrowth in patients with alopecia areata.
Efficacy: Clinical studies have shown that ritlecitinib is an effective treatment for alopecia areata and vitiligo. In a phase 2 study in patients with alopecia areata, oral Ritlecitinib was shown to increase hair regrowth in many patients; 58% of patients achieved SALT 20 (≤20% scalp hair hau), 11% of patients used placebo. In a randomized phase 2b clinical trial in patients with non-segmental vitiligo, oral Ritlecitinib was shown to be effective in inducing repigmentation, and a significant proportion of patients achieved remission with a Vitiligo Area Score Index (VASI) of 50 (≥50% improvement in VASI score). compared with placebo. These results suggest that ritlecitinib has the potential to be very effective in the treatment of these diseases.
Safety: In clinical studies, ritlecitinib was generally well tolerated and no adverse effects occurred. The most common side effects are upper respiratory tract infection, headache and diarrhea, which are mild to moderate and do not improve with additional treatment. This suggests that ritecitinib is an effective treatment for patients with alopecia areata and vitiligo.
Taken together, ritlecitinib has many advantages as a treatment for alopecia areata and vitiligo, including selective inhibition, clinical efficacy, efficacy, and safety. These positive results make Ritlecitinib a good treatment option for patients with this disease, but more research is needed to determine its long-term benefits. Overall, ritecitinib is promising as a treatment option for alopecia areata and non-segmental vitiligo, with advantages of selective inhibition, clinical efficacy, efficacy, and safety. However, more research is needed to determine its long-term benefits.
Disadvantage of LITFULO (ritecitinib)
While Ritlecitinib has many advantages as a treatment option for alopecia areata and vitiligo, it has some disadvantages to consider:
Cost: Ritlecitinib may be more expensive than other treatments for alopecia areata and vitiligo, according to a new study. This can be a disadvantage for patients who do not have insurance or cannot afford medication.
Long Term: While Ritlecitinib shows promise in promoting hair regrowth and repigmentation in clinical trials, more research is needed to determine its long-term effects. This medicine may have unknown side effects or may lose its effectiveness over time.
Adverse events: Although ritlecitinib was generally well tolerated in clinical trials, some patients may experience adverse events such as upper respiratory tract infections, headaches and stomach diarrhoea.These side effects are mild to moderate and resolve without further treatment, but may still be fatal in some patients.
Limited indications: Ritlecitinib is currently only indicated for the treatment of alopecia areata and non-segmental vitiligo. This means it may not work on other hair or skin types, and this can be harmful for patients with these conditions.
In conclusion, while Ritlecitinib has many advantages as a treatment for alopecia areata and vitiligo, there are also some disadvantages to consider, such as cost, long-term effects, poor condition, and few symptoms. Patients should discuss these factors with their doctor to determine if Ritlecitinib is an appropriate treatment for them.
Market Growth and Size of Alopecia Areata
The alopecia areata treatment market is growing and is expected to continue to grow in the coming years. Here are some details about market size and growth:
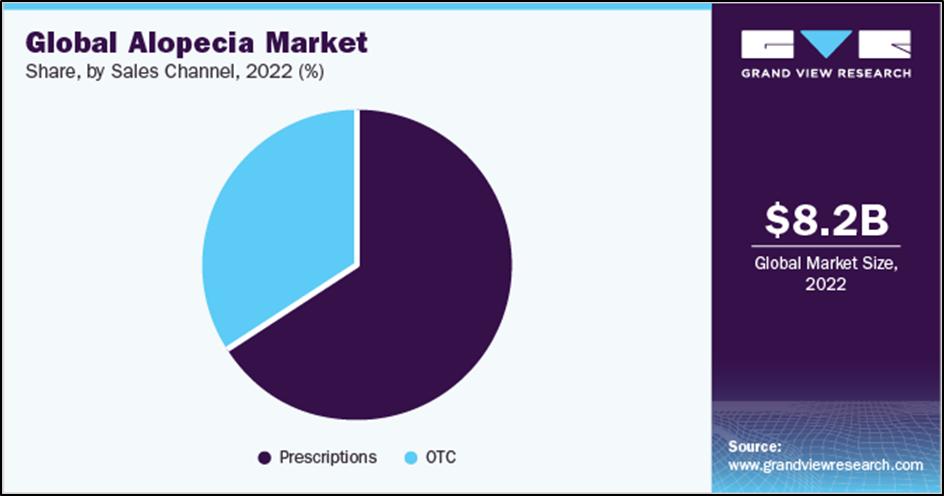
The global hair loss market will be worth $8.25 billion in 2021. With a compound annual growth rate (CAGR) of 7, it is expected to reach $13.7 billion by 2030.7% of the estimated time. This shows that the market has growth potential.
Factors contributing to the growth of hair loss include the increased incidence of hair loss due to chronic diseases, developments in hair care technology, and increasing hair loss. These conditions increase the need for effective treatment options. The market has seen advances in hair treatment such as the approval of JAK inhibitors and tyrosine kinase inhibitors, expanding existing treatments.With the increasing number of diseases, these developments are expected to drive the growth of the industry.
Increasing awareness of hair loss and the desire to improve their appearance have led many people to seek hair treatment. This trend has contributed to the growth of the economy.Market growth is also driven by investment and financing in research and development (R&D) for hair care. These investments encourage innovation and facilitate business expansion.However, the market is facing challenges such as the limitation of drugs aimed at treating the underlying cause of hair loss. While new products are approved for study purposes, the high cost of these drugs relative to recommended treatments can be a deterrent for some patients.
In conclusion, the alopecia areata treatment market is growing and should continue. Factors such as the increase in the incidence of chronic diseases related to hair loss, technological developments, the increase in the incidence of hair loss and the increased awareness of people seeking treatment led to economic growth. However, challenges remain, such as limited supply and the high cost of targeted drugs.
Global Alopecia Market
Pictorial Representation of Alopecia Areata Drug
Due to the large number of dermatologist visits for hair treatment, the current sales of the hair loss market are drug-heavy with a share of 66.2%. According to the U.S. National Library of Medicine, approximately 2.6 million outpatients in the United States visit dermatologists for alopecia areata. Additionally, strong products, increasing disposable income and increasing incidence of chronic hair loss are expected to drive the fastest price segment growth during the forecast.
However, OTC trading continues, the segment is widely used in the creation of regions where players can find more opportunities to address the unmet medical needs of large numbers of patients in countries such as China, Japan, Mexico, Brazil and India. Additionally, over-the-counter drugs are still the drug of choice for men with androgenetic alopecia and other types of hair loss, as there are no approved drugs, vaccines, or over-the-counter drugs that give consumers hope.
Global Market Forecast and Market Penetration
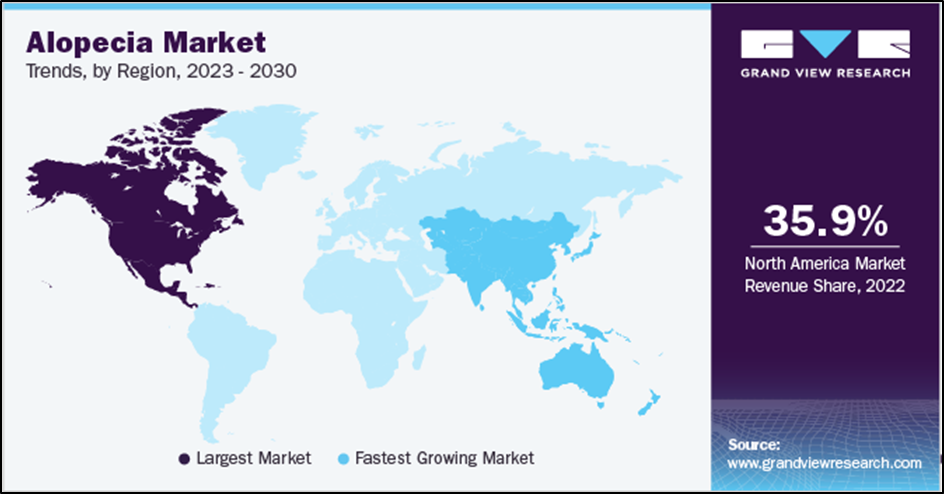
North America holds the leading region in the Hair Care market with a share of 35.89% in 2022, mainly due to the increasing number of diseases, increased consumer awareness, government employment measures, healthcare infrastructure and technology development. In addition, the increasing use of laser hair loss treatment also contributes to the progress of treatment in North America. In addition, government and non-profit organizations such as the National Alopecia Areata Foundation (NAAF), Canadian Alopecia Foundation provide advice, and more.
Alopecia Areata Market
Asia Pacific is expected to record the highest CAGR of over 9.5% during the forecast period. The prevalence of hair loss is increasing in many Asian countries, leading to the demand for effective treatment. Awareness of hair loss and its impact on quality of life is also driving the growth of the market. In addition, the increase in people’s incomes and changes in lifestyle have made people willing to spend money on hair treatment.In addition, improving environmental health management in developing countries should attract investment from international players and take advantage of already existing business opportunities. For example, in 2016, China’s regulatory agency, the State Food and Drug Administration, approved Apira Science’s iGrow laser system, making it the first laser hair growth device approved in China.
Europe is also expected to be an important market during the forecast period. This growth can be attributed to many factors, such as the development of technology, advertisements becoming known to public and private groups. The European Hair Research Institute plays an important role in conducting hair research-related activities and providing information on effective treatments to protect hair.
Overall Development in Alopecia Market
| Report Attribute | Details |
| Market size value in 2023 | USD 8.77 billion |
| Revenue forecast in 2030 | USD 16.02 billion |
| Growth rate | CAGR of 9.0% from 2022 to 2030 |
| Base year for estimation | 2022 |
| Historical data | 2018 – 2021 |
| Forecast period | 2022 – 2030 |
| Report updated | June 2023 |
| Quantitative units | Revenue in USD million and CAGR from 2022 to 2030 |
| Report coverage | Revenue forecast, company ranking, competitive landscape, growth factors, and trends |
| Segments covered | Disease type, treatment, gender, sales channel, end-use, region |
| Regional scope | North America; Europe; Asia Pacific; Latin America; MEA |
| Country scope | U.S.; Canada; UK; Germany; Spain; France; Italy; Denmark; Sweden; Norway; India; China; Japan; Australia; South Korea; Thailand; Brazil; Mexico; Argentina; Saudi Arabia; South Africa; UAE; Kuwait |
| Key companies profiled | Johnson & Johnson Services, Inc; Merck & Co., Inc.; Cipla Limited; Sun Pharmaceuticals Industries Ltd; Dr. Reddy’s Laboratories Ltd; GlaxoSmithKline plc.; Aurobindo Pharma; Viatris Inc.; Pfizer, Inc.; Lilly; Lexington Intl., LLC (Devices); Freedom Laser Therapy (iRestore ID-520 helmet); Curallux, LLC.; Apira Science Inc. (iGROW Laser); Revian Inc.; Theradome; Lutronic |
There have been various developments in the Allopecian Market Recently and several multinational companies have taken initiatives to find the cure for the multibillion dollar Disease Market .
Key Players in the Alopecia Areata Market
In March 2023, the international pharmaceutical company Sun Pharmaceuticals Industries Ltd. Clinical data published from Phase 3 clinical trial of deruxolitinib, an oral drug that improves scalp hair regrowth in patients with alopecia areata
June 2023, Pfizer Biotechnology Multinational. LITFULO (ritlecitinib) is approved by the U.S. Food and Drug Administration (FDA) as an oral treatment for severe alopecia areata in patients 12 years of age and older. This approval makes LITFULO™ an advanced treatment for people with alopecia areata.
On June 2022, Eli Lilly and Company and Incyte announced U.S. Food and Administration approval of OLUMIANT, a daily pill (FDA) for the treatment of adult alopecia areata. . Patients with complete or near total hair loss should receive 4 mg daily to maintain hair growth
June 2022, Revian Inc.KNOW Bio LLC, a subsidiary of Medical Technology Innovation Company, Central Centrifugal Scarred Alopecia (CCCA)
September 2021 Dr. Reddy’s Laboratories Ltd. The leading Multinational Pharmaceutical Organization in advanced medicine. male pattern baldness
| Manufacturer | Drug |
| Sun Pharmaceuticals Industries Ltd. | Deuruxolitinib (CTP-543) |
| Concert Pharmaceuticals | Deuruxolitinib (CTP-545) |
| Eli Lilly and Company and Incyte | Olumiant (baricitinib) |
| Pfizer Biotechnology Multinational | Ritlecitinib (LITFULOTM) |
| Revian Inc. and KNOW Bio LLC | N/A |
Conclusion
In conclusion, the advertise investigate conducted on ritlecitinib for the treatment of alopecia areata uncovers profoundly promising comes about and considerable potential for development. The clinical trials and considers conducted hence distant illustrate the viability of ritlecitinib in actuating critical hair regrowth, whereas too highlighting its favorable security profile. The specific hindrance and systemic treatment advertised by ritlecitinib position it as a exceedingly promising treatment choice for alopecia areata and nonsegmental vitiligo, tending to the neglected needs of patients in these helpful areas.
Moreover, the showcase potential for ritlecitinib is significant, with projections demonstrating deals surpassing $1 billion within the alopecia showcase alone. This underscores the critical request and showcase opportunity for an progressed treatment alternative like ritlecitinib. Besides, the expected development of the alopecia areata showcase advance reinforces the positive viewpoint for ritlecitinib’s victory and showcase penetration.
However, it is critical to note that assist inquire about is required to completely get it the long-term impacts and potential impediments of ritlecitinib. Proceeded examination and observing of its viability, security, and tolerability will be significant to guarantee its supported victory and acknowledgment inside the therapeutic community.
In rundown, ritlecitinib illustrates gigantic guarantee as an progressed treatment alternative for people with alopecia areata. Its showcase viewpoint shows up profoundly favorable, driven by its demonstrated adequacy, favorable security profile, and the developing demand for inventive treatments within the field of alopecia treatment. As ritlecitinib proceeds to experience advance investigate and advancement, it has the potential to essentially affect the lives of patients and ended up a driving player within the alopecia treatment advertise.
References
- ClinicalTrials.gov. A Study to Learn About the Study Medicine (Called Ritlecitinib) For the Potential Treatment of Severe Alopecia Areata (AA) In Children 6 To Less Than 12 Years of Age – Clinical Trials. Accessed on December 14, 2022. Available at: https://clinicaltrials.gov/ct2/show/NCT05650333
- ResearchGate. Evaluating the Therapeutic Potential of Ritlecitinib for the Treatment of Alopecia Areata. Accessed on February 1, 2023. Available at: https://www.researchgate.net/publication/358883144_Evaluating_the_Therapeutic_Potential_of_Ritlecitinib_for_the_Treatment_of_Alopecia_Areata
- PubMed Central. Evaluating the Therapeutic Potential of Ritlecitinib for the Treatment of Alopecia Areata. Accessed on February 17, 2022. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8860347/
- Journal of Allergy and Clinical Immunology. Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers. Accessed on December 1, 2021. Available at: https://www.jacionline.org/article/S0091-6749(21)01823-6/fulltext
- ScienceDirect. Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers. Accessed on December 1, 2021. Available at: https://www.sciencedirect.com/science/article/pii/S0091674921018236
- https://www.grandviewresearch.com/industry-analysis/alopecia-market
- https://www.delveinsight.com/report-store/alopecia-areata-market
- https://www.alliedmarketresearch.com/alopecia-treatment-market-A05945
- https://www.persistencemarketresearch.com/market-research/alopecia-treatment-market.asp
- https://www.verifiedmarketresearch.com/product/alopecia-market/






Leave a Reply