Lantidra (donislecel), an allogeneic cellular therapy, has recently obtained approval from the usa meals and Drug management (FDA) for the remedy of kind 1 diabetes. This groundbreaking approval, announced on June 28, 2023, marks a massive milestone because the first approval of an allogeneic pancreatic islet mobile therapy for type 1 diabetes, kind 1 diabetes is a chronic situation characterised by means of the frame’s incapability to supply insulin, requiring individuals to depend on external insulin management.
Lantidra gives a brand new technique to dealing with kind 1 diabetes with the aid of making use of allogeneic pancreatic islet cells derived from deceased donors.This modern therapy pursuits to deal with the needs of adults with type 1 diabetes who experience repeated episodes of intense hypoglycemia despite intensive diabetes control and training, and are not able to obtain goal glycated hemoglobin degrees.
The approval of Lantidra follows a thorough assessment system through the FDA’s cellular, Tissue, and Gene treatment plans Advisory Committee, which concluded with a 12-4 vote in prefer of the clinical price of donislecel. This approval indicates the popularity of the remedy’s ability benefits and its chance-gain profile.
Lantidra’s approval opens new possibilities for the remedy of type 1 diabetes, providing desire for people who struggle to attain glycemic manipulate despite modern-day management strategies. through utilising allogeneic pancreatic islet cells, Lantidra gives a novel method to restoring insulin manufacturing and improving glucose law in people with type 1 diabetes
The approval of Lantidra additionally highlights the advancements in cellular treatment plans and their ability to revolutionize the remedy landscape for kind 1 diabetes. as the first-ever cell remedy approved for type 1 diabetes, Lantidra represents a massive milestone in the subject of regenerative remedy
In summary, the FDA’s approval of Lantidra (donislecel) as an allogeneic cell remedy for kind 1 diabetes marks a groundbreaking improvement within the treatment of this chronic condition. Lantidra offers a new approach to handling type 1 diabetes by means of using allogeneic pancreatic islet cells, supplying desire for those who war to achieve glycemic manipulate. This approval showcases the ability of mobile cures in reworking the remedy landscape for kind 1 diabetes and represents a sizeable development in regenerative medicinal drug
Mechanism of Action – Lantidra
The primary mechanism of action of Lantidra (donislecel) is believed to be insulin secretion by infused allogeneic islet beta cells.Pancreatic islets, which are composed of endocrine cells, including beta cells, play a key role in regulating blood glucose levels by releasing hormones such as insulin, glucagon, somatostatin, pancreatic peptide, and ghrelin.
Lantidra uses purified pancreatic cells obtained from deceased donors and transplants them into recipients with fragile type 1 diabetes to supplement endogenous insulin and glucagon production.This transplant aims to improve pancreas-mediated control of blood glucose and potentially eliminate the need for exogenous insulin administration
Infusion of Lantidra into the hepatic portal vein via a percutaneous or transvenous transhepatic approach or a laparoscopic or open surgical approach enables allogeneic islet beta cells to secrete insulin and contribute to glucose regulation
By restoring insulin production, Lantidra offers a new approach to treating type 1 diabetes and potentially improving glycemic control in individuals who experience repeated episodes of severe hypoglycemia despite intensive diabetes treatment and education.
FDA approval of Lantidra for the treatment of type 1 diabetes recognizes its clinical value and the potential benefits it offers to individuals with this chronic disease. This allogeneic cell therapy represents a major advance in the field of regenerative medicine and provides hope for those struggling to achieve glycemic control with current management strategies.
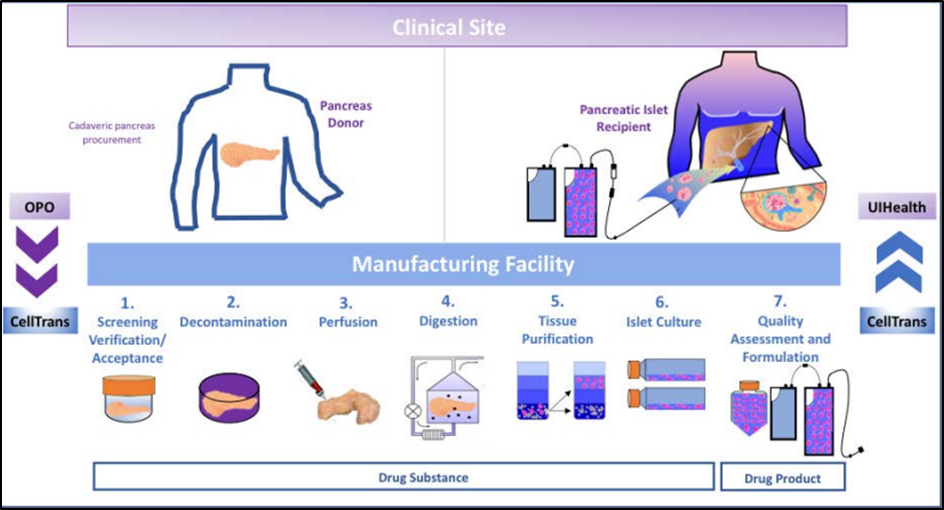
Proposed mechanism
Briefly, Lantidra (donislecel) exerts its therapeutic effect by secreting insulin through infused allogeneic islet beta cells. By supplementing endogenous insulin and glucagon production, Lantidra aims to improve pancreas-mediated blood glucose control and potentially eliminate the need for exogenous insulin administration. This mechanism of action offers a promising approach to the treatment of type 1 diabetes and represents a major milestone in the field of regenerative medicine.
Advantages of Lantidra (donislecel)
Lantidra (donislecel) gives several benefits for the treatment of kind 1 diabetes, along with:
Novel method: Lantidra represents a singular technique to dealing with type 1 diabetes with the aid of making use of allogeneic pancreatic islet cells derived from deceased donors.This progressive remedy targets to cope with the desires of adults with type 1 diabetes who enjoy repeated episodes of excessive hypoglycemia despite intensive diabetes control and training, and are not able to achieve goal glycated hemoglobin tiers
Ability elimination of the want for exogenous insulin administration: Lantidra probably makes it easier for people with type 1 diabetes to control their situation because the infused allogeneic islet beta cells can produce sufficient insulin to govern blood glucose ranges, getting rid of the need for insulin remedy
Progressed glycemic control: by way of supplementing endogenous insulin and glucagon manufacturing, Lantidra objectives to improve pancreas-mediated control of blood glucose and doubtlessly improve glycemic manage in people who experience repeated episodes of extreme hypoglycemia notwithstanding extensive diabetes control and education
Unmarried infusion: Lantidra is administered as a unmarried infusion into the liver portal vein, which may be more handy for sufferers than different treatment options that require frequent injections or infusions
Additional infusion: an additional infusion of Lantidra can be completed depending on the patient’s response to the initial dose, making an allowance for individualized treatment
In precise, Lantidra gives several benefits for the treatment of type 1 diabetes, inclusive of a novel approach to coping with the circumstance, the capacity removal of the need for exogenous insulin administration, stepped forward glycemic manage, a single infusion, and the choice for an additional infusion based totally on the affected person’s response to the preliminary dose. these blessings make Lantidra a promising therapy for people with type 1 diabetes who battle to acquire glycemic manage with contemporary management strategies.
Disadvantages of Lantidra (donislecel)
Risk of infections and cancer: The immune suppression needed to keep the islet cells alive may increase the risk of infections and cancer
Side effects: Common side effects of Lantidra include nausea, fatigue, anemia, diarrhea, and abdominal pain Some participants experienced serious adverse reactions, primarily related to the infusion procedure and the use of immunosuppressive medications
Limitations of use: When considering the risks associated with the infusion procedure and long-term immunosuppression, there is no evidence to show a benefit of Lantidra in patients with type 1 diabetes who have adequate glycemic control with insulin therapy alone
In summary, Lantidra offers a novel approach to managing type 1 diabetes, but it also has some disadvantages. The immune suppression needed to keep the islet cells alive may increase the risk of infections and cancer.
Common side effects of Lantidra include nausea, fatigue, anemia, diarrhea, and abdominal pain. Some participants experienced serious adverse reactions, primarily related to the infusion procedure and the use of immunosuppressive medications.
Additionally, Lantidra has limitations of use, and there is no evidence to show a benefit of the therapy in patients with type 1 diabetes who have adequate glycemic control with insulin therapy alone.
Clinical Phases of Lantidra (donislecel)
Lantidra (donislecel) is an allogeneic cell remedy that has been accepted by the FDA for the remedy of kind 1 diabetes. The remedy’s mechanism of motion includes the secretion of insulin with the aid of the infused allogeneic islet beta cells, which aims to enhance pancreas-mediated manage of blood glucose and potentially cast off the need for exogenous insulin administration. The approval of Lantidra by using the FDA become primarily based on facts from two potential, open-label, single-arm studies that evaluated the efficacy and safety of donislecel in adults with type 1 diabetes and hypoglycemic unawareness. The producer of Lantidra, CellTrans, plans to conduct additional clinical trials to assess the long-time period protection and efficacy of the remedy.
| Year | Description |
| 2018 | The FDA granted Fast Track designation to Lantidra for the treatment of type 1 diabetes |
| 2020 | The FDA granted Breakthrough Therapy designation to Lantidra for the treatment of type 1 diabetes |
| 2021 | The FDA granted Priority Review designation to Lantidra for the treatment of type 1 diabetes |
| 2023 | The FDA approved Lantidra (donislecel) as the first allogeneic pancreatic islet cellular therapy for the treatment of type 1 diabetes |
| Future Steps | The manufacturer of Lantidra, CellTrans, plans to conduct additional clinical trials to evaluate the long-term safety and efficacy of the therapy |
In summary, Lantidra has passed through several clinical trials before its approval because the first allogeneic pancreatic islet mobile therapy for the remedy of type 1 diabetes.
The approval of Lantidra by way of the FDA changed into based totally on data from prospective, open-label, single-arm studies that evaluated the efficacy and safety of donislecel in adults with type 1 diabetes and hypoglycemic unawareness.
The producer of Lantidra plans to conduct extra medical trials to assess the long-term safety and efficacy of the remedy, representing a giant milestone within the area of regenerative medicine.
Market Players of Lantidra (donislecel)

Lantidra (donislecel) is synthetic via CellTrans Inc., a agency based totally in Chicago, USA.The remedy is a pancreatic islet cell therapy made from the pancreatic cells of deceased donors.CellTrans evolved Lantidra as an allogeneic mobile remedy for the remedy of adults with type 1 diabetes.The FDA accredited Lantidra on June 28, 2023, under the logo call Lantidra
The approval of Lantidra by the FDA marks a substantial milestone because the first allogeneic pancreatic islet mobile remedy authorised for the treatment of kind 1 diabetes. The protection and efficacy of Lantidra had been confirmed in scientific trials performed in subjects with kind 1 diabetes and hypoglycemic unawareness
In summary, Lantidra is manufactured with the aid of CellTrans Inc., a organisation based totally in Chicago. The remedy is a pancreatic islet cellular therapy crafted from the pancreatic cells of deceased donors. The FDA has permitted Lantidra because the first allogeneic pancreatic islet cell remedy for the treatment of kind 1 diabetes. The therapy’s approval represents a substantial development inside the discipline of regenerative medication.
Market Size of Type 1 Diabetes
Type 1 Diabetes market size varies from region to region, but all will grow in the coming years. According to a report by Precedence Research, the global diabetes market is expected to reach $37.22 billion by 2032 and total revenue growth is expected to be $100 million from 2023 to 2038.
17MM (Seven Markets) is expected to undergo major changes in the 2019-2032 operating period. According to IMARC Group, the size of India’s diabetes market is $3.
| Aspects | Details |
| Market Size By 2030 | USD 317.9 billion |
| Growth Rate | CAGR of 10.4% |
| Forecast period | 2020 – 2030 |
| Report Pages | 160 |
| By Product | Injectables Oral antidiabetic Drugs |
| By Region | North America (U.S., Canada, Mexico) Europe (Germany, France, United Kingdom, Italy, Spain, Rest of Europe) Asia-Pacific (China, Japan, India, Australia, Rest Of Asia Pacific) LAMEA (Brazil, Saudi Arabia, South Africa, Rest of LAMEA) |
| Key Market Players | BOEHRINGER INGELHEIM GMBH, SANOFI S.A., ASTRAZENECA PLC, ELI LILLY AND COMPANY, NOVO NORDISK, MERCK and CO., INC., NOVARTIS AG, TAKEDA PHARMACEUTICAL COMPANY LIMITED, johnson and johnson md&d, GLAXOSMITHKLINE PLC |
Growing Graph of Antidiabetics
The global diabetes treatment market is expected to have a bright future and the market size is expected to reach $317.9 billion by 2030, with a CAGR of 10.4% from 2020 to 2030. Work should be guided by the increase in diabetes and a sedentary lifestyle. Lifestyle and other chronic diseases such as hypertension.The market is segmented into injectables and oral contraceptives by product and North America, Europe, Asia Pacific and LAMEA by region, AstraZeneca, Eli Lilly, Novo Nordisk, Merck, Novartis, Takeda Pharmaceuticals, Johnson & Johnson MD&D and GlaxoSmithKline
Increasing Diabetic Population, Technological Innovation and Increasing Adoption Developing regions are key drivers of growth in the world’s largest diabetes market.
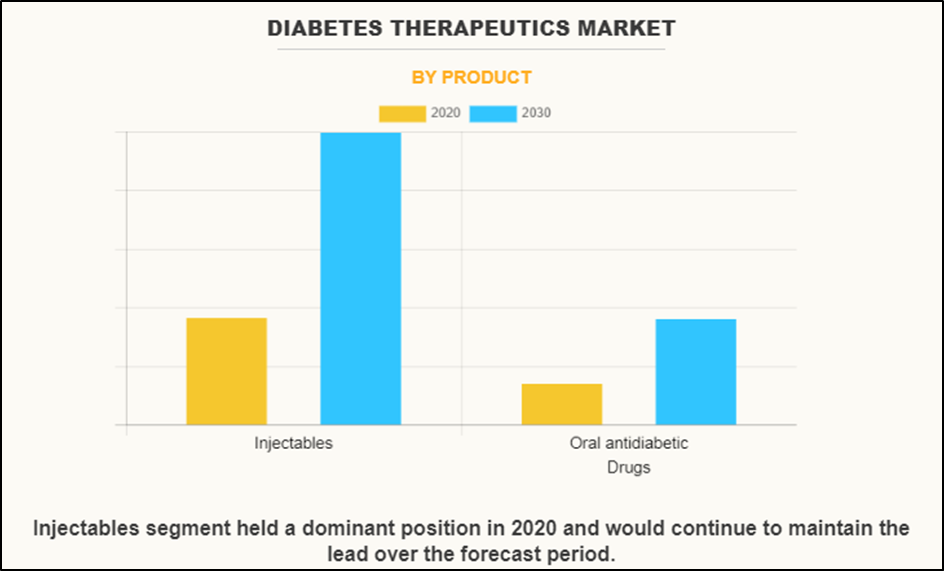
Graph Depicting Increase in Antidiabetic Drugs
Future of Lantidra with respect to Antidiabetic Market
Based on available data, the future of antidiabetic drugs on the diabetes market looks bright, including Lanitdra. Below are some key facts that support this:
Market Size: The global diabetes treatment market is expected to reach $317.9 billion by 2030, showing strong growth potential.
Growth: The market is projected to grow at a CAGR of 10.4% between 2020 and 2030, indicating steady, positive growth.
Increasing Prevalence: The prevalence of diabetes worldwide is increasing and more and more people are being diagnosed with the disease.
Technological Innovation: The market is driven by technological advances and innovations in the treatment of diabetes, including the development of new drugs and treatment options.
Key Players: Lanitdra is involved in the diabetes treatment industry along with other key players such as Boehringer Ingelheim Ltd, Sanofi, AstraZeneca and Novo Nordisk.
Growing Healthcare Market: Rising global healthcare costs are expected to help the diabetes care market grow.
Access to Medicine: Increasing access to insurance and the affordability of medicines, making vaccines affordable for more people around the world. Overall, anti-diabetic drugs like Lanitdra may have a future in the diabetes market with the rise in diabetes, technological advances and good business. However, it’s worth noting that more research and clinical trials are needed to evaluate Lanitdra’s efficacy and commercial potential in the treatment of diabetes.
Conclusion
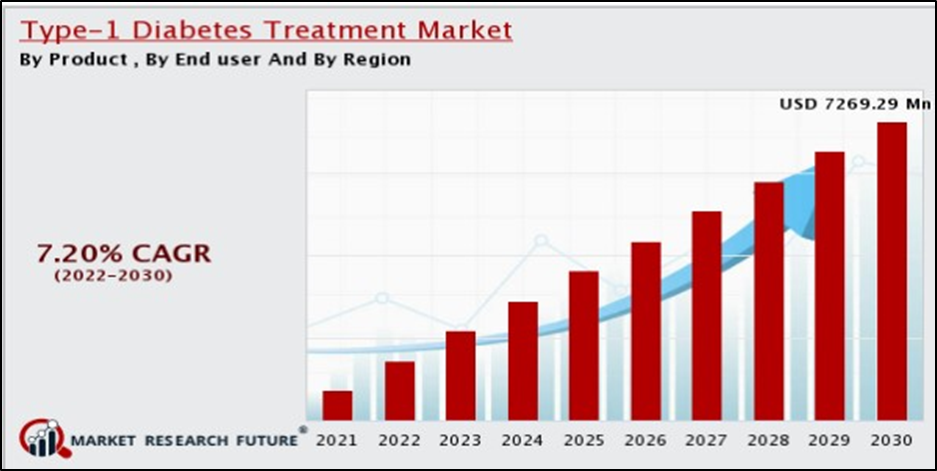
Lantidra’s market research report shows that there is hope for this anti-diabetic drug in the diabetes market. The report provides comprehensive information on the market size, growth rate and key players of the global Diabetes Treatment market. The market size is expected to grow at a CAGR of 10.4% from 2020 to 2030, reaching $317.9 billion by 2030.
The report also highlights the increasing prevalence of diabetes, technological advances and rising healthcare spending as key drivers of market growth. It segments the market for oral contraceptives by product and into North America, Europe, Asia Pacific and Latin America by region. Major companies include Boehringer Ingelheim, Sanofi, AstraZeneca, Eli Lilly, Novo Nordisk, Merck & Co., Inc., Novartis AG, Takeda Pharmaceuticals Inc. Ltd., Johnson & Johnson MD&D, and GlaxoSmithKline PLC.
While the report does not provide specific information about Lantidra, it does suggest that the future of antidiabetic drugs in the diabetes industry is bright. The report shows that the increasing number of diabetes patients, technological advances and market approval are the main factors driving the growth of the diabetes drug market in the world.
Overall, Lantidra’s market research report provides a valuable view for the Diabetes Care industry, highlighting the industry’s potential for growth and innovation.The findings of this report can be used to inform marketing strategies and production plans for Lantidra and other antiretroviral drugs on the market.
References
- Global Diabetes Drugs Market Size, Share, Growth, Trends, Forecast 2021-2028 – Fortune Business Insights
- Diabetes Drugs Market Size, Share, Growth, Trends, Forecast 2021-2028 – Data Bridge Market Research
- Diabetes Drugs Market Size, Share, Growth, Trends, Forecast 2021-2028 – Grand View Research
- Diabetes Drugs Market Size, Share, Growth, Trends, Forecast 2021-2028 – Market Research Future
- Diabetes Drugs Market Size, Share, Growth, Trends, Forecast 2021-2028 – Zion Market Research
- Breaking News: FDA Approves Lantidra for Treatment of T1D. What Does this Mean for a T1D Practical Cure? – Juvenile Diabetes Cure Alliance (JDCA): This article discusses the FDA approval of Lantidra for the treatment of type 1 diabetes and its implications.
- New Type 1 Diabetes Treatment Eliminates Need for Insulin – Verywell Health: This article highlights the FDA approval of Lantidra as a new drug for managing low blood sugar in individuals with type 1 diabetes.
- Raji Akileh, DO on LinkedIn: FDA approval for Lantidra: This LinkedIn post announces the FDA approval of Lantidra for use in cell therapy.
- Pen Needles Market Size, Share, Scope, Trends, Opportunities & Forecast – Verified Market Research: While not specific to Lantidra, this report provides a holistic evaluation of the pen needles market, which is relevant to the delivery of diabetes treatments.






Leave a Reply