Dostarlimab is a monoclonal antibody medication that targets the programmed death receptor-1 (PD-1) and is sometimes referred to as TSR-042 or JNJ-63723283. It is applied in the management of several forms of cancer.
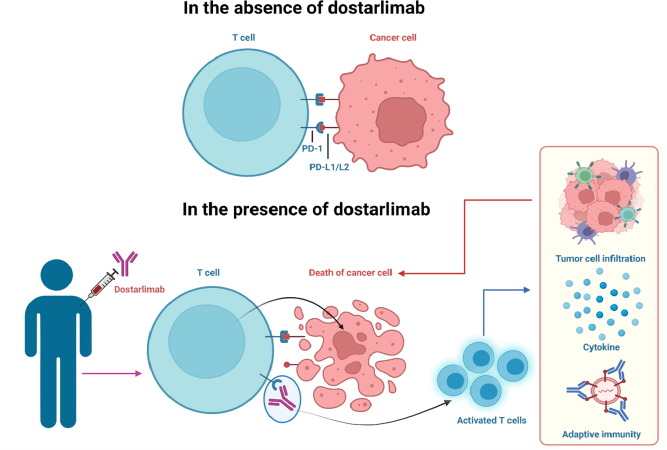
Dostarlimab functions by preventing communication between PD-1 and PD-L1, and PD-L2, its ligands. While PD-L1 and PD-L2 are proteins expressed on the surface of cancer cells, PD-1 is a checkpoint protein located on the surface of immune cells. Dostarlimab aids in restoring the immune system’s capacity to recognise and combat cancer cells by preventing the PD-1/PD-L1 interaction.
Targeted Use of Dostarlimab Injection
Regulators have given Dostarlimab the go-ahead to treat advanced or recurrent mismatch repair-deficient (dMMR) endometrial cancer. The DNA repair process is faulty in dMMR tumours, which results in a larger mutation burden and more PD-L1 expression. It can aid in triggering the immune system to attack these cancer cells by specifically targeting PD-1. Also, in 2022 it received approval for the treatment of endometrial cancer in both the European Union and the United States. AnaptysBio originally created dostarlimab, which was eventually licenced to GSK (NYSE:GSK) under the brand name ‘Jemperli‘.
Additionally, it is being researched for use as both a monotherapy and as a component of combination regimens, for patients with other advanced solid tumours or metastatic cancers, as well as for women with recurrent or primary advanced endometrial cancer, women with stage III or IV non-mucinous epithelial ovarian cancer, and women with recurrent or primary advanced endometrial cancer.
In a clinical trial funded by GSK, the National Cancer Institute of the NIH, Stand Up to Cancer, the Simon and Eve Colin Foundation, Swim Across America etc had some positive results. Twelve individuals with rectal cancer were entirely cured of their disease after using a medication for six months in a medical clinical trial that is still ongoing. Following administration, these patients underwent a battery of tests, including physical inspection, endoscopy, bioscopy, PET scans, and MRI scans, all of which revealed no evidence of tumours.
Dostarlimab’s efficacy in adjuvant or neoadjuvant therapy, or in early stages of cancer, is still being investigated. Neoadjuvant therapy is administered prior to the initial treatment to reduce tumour size and enhance surgical results, whereas adjuvant therapy seeks to lower the chance of cancer recurrence following primary treatment.
It is important to note that this injection may have potential adverse effects, which might vary from person to person as with any medication. Fatigue, diarrhoea, rashes, itching, and modifications in liver function are examples of frequent adverse reactions. Although they are uncommon, immune-related adverse events like pneumonitis, colitis, hepatitis, and endocrinopathies can have serious side effects.
It’s crucial to remember that these conceivable uses for the future are founded on active research and clinical testing. The results of these studies and regulatory approvals will determine the creation and approval of additional Dostarlimab indications.
Pharmaceutical Reach
Growth Plus Reports conducted a market research and projected an anticipated revenue CAGR of 15.6% from 2023 to 2031. Dostarlimab-gxly’s global market analysis suggests that the revenue share is projected to increase significantly over the forecast period. Dostarlimab-gxly, a monoclonal antibody medication also known as Jemperli, is used to treat some malignancies.
The market revenue share is being driven by the rising number of patients with endometrial cancer or advanced cancer. Regulatory clearances enable market entry and improved patient accessibility. With the drug’s approval, a significant unmet medical need is now being met, which will aid in the expansion of the dostarlimab-gxly market.
The dostarlimab-gxly market is divided into endometrial cancer, skin cancer, lung cancer, liver cancer, kidney cancer, and others based on the kind of cancer. The dostarlimab-gxly market is divided into hospitals, homecare settings, ambulatory surgery centres, and others based on end users.
Based on region, the dostarlimab-gxly market is dominated by North America, which has the highest revenue share. The high cost of healthcare, the established healthcare system, the rising prevalence of cancer, and the favourable reimbursement conditions are all attributed to this high income share.
In the future, if the drug is shown to be highly effective in research, one crucial consideration is the cost. The cost per dose is approximately $11,000 in the US. Due to the fact that 70% of all cancers are predicted to occur in low- and middle-income nations, the drug’s price must decrease in order for it to be affordable to a sizable population who is suffering from the disease.
Several patents provide protection for Dostarlimab (TSR-042 or JNJ-63723283). Tesaro, the original company that created Dostarlimab, was principally responsible for the patents in this medication.
Tesaro and Janssen (a pharmaceutical firm that is a member of the Johnson & Johnson family), worked together to develop and market Dostarlimab. They have the rights to create, produce, and commercialise Dostarlimab. Dostarlimab’s development and clinical trials for a few indications were also helped by MacroGenics.
GlaxoSmithKline (GSK) now owns the Dostarlimab patents after acquiring Tesaro in 2019. GSK continued the investment in Dostarlimab as part of its oncology portfolio.
Due to intensive research and increased healthcare spending globally, the market situation for metastatic colorectal cancer is expected to change. This would allow drug manufacturers to enhance their market share.
The companies are working on therapies that examine problems and look for opportunities that could affect the dominance of JEMPERLI (dostarlimab) in order to treat or improve the medical condition.
JEMPERLI (dostarlimab) is anticipated to face stiff competition from other emerging medications for metastatic colorectal cancer, and the impending introduction of late-stage emerging therapies will have a big impact on the market.






Leave a Reply