
BIOCON-VIATRIS SEMGLEE GETS USFDA APPROVAL AS FIRST INTERCHANGEABLE BIOSIMILAR PRODUCT
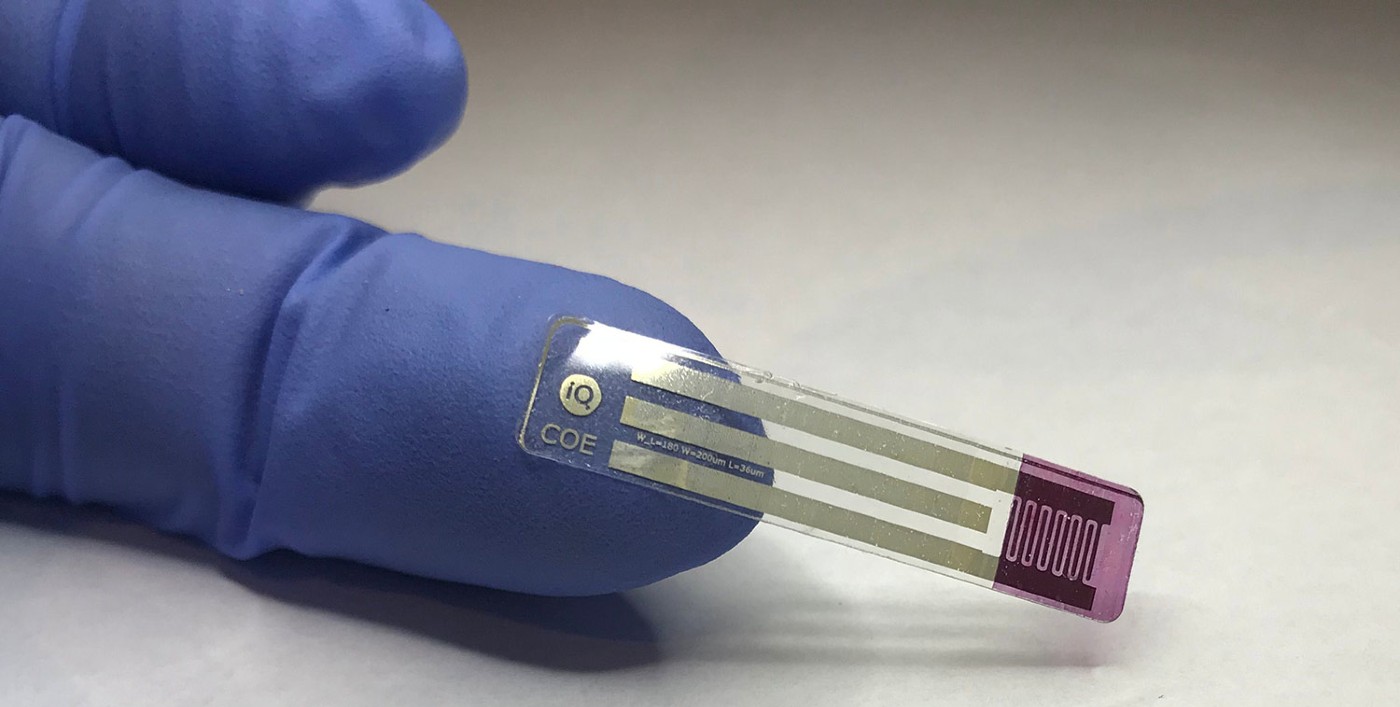
NEEDLE FREE BLOOD SUGAR TEST

MODERNA TO USE mRNA FOR HIV AND CANCER AFTER COVID-19
LAMBDA : NEW VARIANT WITH 7 MUTATIONS
INDIA ADMINISTERED OVER 32 CRORE DOSES, OVERTAKES US
NINE EUROPEAN COUNTRIES APPROVE INDIAN VACCINE COVISHIELD FOR GREEN PASS

ZYDUS CADILA’S VACCINE TO BE JUST AS EFFECTIVE WITH A TWO- DOSE REGIMEN

SII TO SUPPLY 20 CRORE COVAVAX DOSES BY DEC 2021
FIRST DEATH FOLLOWING COVID-19 VACCINATION



